We certify that we have read this thesis and
that in our opinion it is satisfactory in scope and quality as a thesis for the
degree of Master of Science in Agronomy.
|
|
ROOT
TUBERIZATION AND NITROGEN FIXATION
BY
PACHYRHIZUS EROSUS (L.)
A THESIS SUBMITTED TO THE
GADUATE DIVISION OF THE
UNIVERSITY OF HAWAII IN
PARTIAL FULFILLMENT
OF THE REQUIREMENTS FOR
THE DEGREE OF
MASTER
OF SCIENCE
IN
AGRONOMY
MAY
1979
By
Paul
Lester Woomer
Thesis
Committee:
A. Sheldon Whitney,
Chairman
B. Ben Bolhool
Peter Rotar
Wallace Sanford
We certify that we have read this thesis and
that in our opinion it is satisfactory in scope and quality as a thesis for the
degree of Master of Science in Agronomy.
|
|
TABLE
OF CONTENTS
Page
ACKNOWLEDGMENTS
...........................................
4
LIST OF TABLES
............................................
5
LIST OF FIGURES
...........................................
6
LIST OF APPENDICES
........................................
8
CHAPTER I. INTRODUCTION
............................... 9
CHAPTER II. LITERATURE REVIEW
.......................... 12
CHAPTER III. THE RHIZOBIUM AFFINITIES OF
PACHYRHIZUS EROSUS (L.) .................... 31
CHAPTER IV. DIURNAL CHANGES IN SYMBIOTIC
NITROGENASE ACTIVITY OF THE
TUBEROUS-ROOTED LEGUMES
PACHYRHIZUS EROSUS (L.) AND
PSOPHOCARPUS TETRAGONOLOBUS
(L.) DC .................................... 42
CHAPTER V. ACCUMULATION AND PARTITIONING
OF DRY MATTER IN PACHYRHIZUS
EROSUS (L.)
................................ 64
CHAPTER VI. THESIS SUMMARY
............................. 85
CHAPTER VII. LITERATURE CITED
........................... 87
APPENDICES
................................................ 93
ACKNOWLEDGEMENTS
I wish to acknowledge Dr.
Karl Stockinger, Dr. Padmanabhan Somasegaran, Tom Ohara, Scott Mawson and Bruce
Martin for their technical assistance.
Barbara Bird’s
computerized literature services and Sandra Sillapere’s command of the
typewriter are greatly appreciated.
LIST OF TABLES
Table
Page
1 Designation, source
and rating of Rhizobium
strains tested on P. erosus
........................... 34
2 Properties of P. erosus in
response to symbiotic
effectiveness and nitrogen form
....................... 35
3 Regression matrix of plant dry weight and
nitrogen parameters
................................... 39
4 Regression matrix comparing relative
effectiveness
and tuberous root characters
.......................... 40
5 Daily nitrogenase levels of two
tuberous-rooted
legume species
........................................
49
6 Nitrogenase
levels as affected by root and air
temperature
...........................................
50
7 Specific activity of P.
erosus root nodules
as a function of
propagule and sampling time
of day
................................................ 51
8 Components of yield increase of P. erosus
as a function of
propagule ............................
52
9 Acetylene reduction
and root tuberization of
field grown P. erosus
................................. 55
10 Effect of prolonged
darkness on symbiotic
nitrogenase activity
.................................. 56
11 Ratio of maximum and
minimum observed nitrogenase
activities for some
field grown and tuberous
rooted legumes
........................................
58
12 Fluctuation in nitrogenase activity for Vigna
unguiculata and P. erosus ............................. 62
13 Effects
of flower removal upon P. erosus .............. 78
14 Fresh tuberous root
yields after 15 weeks as
affected by
inflorescence removal .....................
84
LIST OF FIGURES
Figure
Page
1 Tuberous
root and root nodules of P. erosus
a) attachment of large
root nodule to root system
b)
interior of root nodule, red region is the
active
bacteroidal zone ............................
15
2 Diurnal
changes in nitrogenase activity of
field
grown soybeans (Glycine max (L.) Merr.) ...... 24
3 Conflicting
reports of diurnal nitrogenase
activity in Lupinus
luteus (L.) ....................
24
4 Diurnal nitrogenase activity of pea (Pisum sativum
(L.)) .............................................. 26
5 Tuberous
root size and shape as a function of
Rhizobium
strain effectiveness .....................
36
6 Vessels
and plants for non-destructive acetylene
reduction assay in the
greenhouse .................. 46
7 P.
erosus (Tpe-1) at the time of sampling
for non-destructive
acetylene reduction ............ 46
8 Diurnal
changes in symbiotic nitrogenanse activity
of
field grown P. erosus at different stages of
tuberous-rootedness
................................ 54
9 Field
experiment at the NifTAL Project site,
P.
erosus 5 weeks after emergence, Vigna
unguiculata
had been recently planted in rows
vacated by the week 3
sampling ..................... 66
10 Dry
matter distribution of field grown P. erosus
over time follows phasic
partitioning .............. 68
11 Nitrogen
accumulation of the components of total
yield
over time, podfill is a strong sink for
available
nitrogen .................................
71
12 Percentage
nitrogen in the tissues of plant
components
over time ...............................
73
13 Rates
of nitrogen accumulation and acetylene
reduction
by field grown P. erosus over time ....... 74
14 Nodule
mass (a) and specific nitrogenase
activity
(b) of field grown P. erosus
over
time .......................................... 75
15 Spacial
displacement of the early root nodules
of
P. eruosus by the tuberous root ................. 76
16 Root
nodule growth and development of field
grown P. erosus
.................................. 76
17 Effects of
flower removal on field grown
P. erosus, flowers removed (left), control
(right) .......................................... 79
18 Effects of deflowering P. erosus
................. 79
19 Extremes of tuberous root cracking. a) minor
cracking
of secondary tuberous root
b)
extreme cracking .............................. 82
20 Prolific lenticel development on the tuberous
root of deflowered treatment (left), control
(right) .......................................... 83
LIST OF APPENDICES
Appendix
Page
1 Productivity of root crops in Hawaii ................. 93
2 Effects of Rhizobium strain on the
components
of yield and nitrogen content of P.
erosus ........... 94
3 Dry matter and nitrogen accumulation for
V. unguiculata and P.
erosus after 8 weeks
of growth in the field ............................... 95
4 Nodule mass and specific nitrogenase
activity
of field grown P. erosus
over time ................... 96
5 Effect of ethylene incubation on dry matter
production of P. erosus
using tuberous roots
as propagules ........................................ 97
CHAPTER
I
INTRODUCTION
Recently Pachyrhizus
erosus (L.) (the Mexican yam bean) has been described as a legume of
under-exploited potential in the tropics by the National Academy of Science (in
press). Although root and tuber crops
tend not to be agricultural export items (Leslie, 1967), this crop is currently
exported from Mexico to the United States (Kay, 1973).
Earlier reports (Bautista
and Cadiz, 1967; Kay, 1973) on the culture of this crop recommended use of
nitrogenous fertilizers and failed to mention that this is a nodulated
legume. More recently Marcarian (1978)
recognized this as a symbiotic legume and considered the description of this
crop’s potential to fix nitrogen in the field to be a current research
goal. This line of research could
reduce the use of costly nitrogenous fertilizers.
The tuberous root of P.
erosus is edible either raw or cooked.
In Hawaii it is called the “Chinese potato” or the “chop suey yam”
(Ezumah, 1970) and is raised on a back yard scale. Determining the yield potential, the optimal time to harvest and
developing management techniques to increase yield and nutritive quality of
this crop could serve to increase production in Hawaii, and potentially develop
an agricultural export commodity at a time when production of sugar cane, the
major crop in the islands, is proving unprofitable without subsidy from the
federal government.
Increased production in
developing tropical countries of this crop as an export commodity to the more
developed countries would have two major consequences. Firstly, revenue would be generated in the
producing countries. Secondly, just as
more protein is needed in the diets of people in the lesser developed
countries, so are less calories needed in the diets of ever fattening affluent
populations. If crispy snack foods can
be processed from P. erosus, these would compete directly with
far more fattening substitutes (cookies, potato chips, peanuts, etc.)
The intent of this thesis
is to describe the sink capacities for assimilate and nitrogen of the various
plant organs of P. erosus.
The following investigations were undertaken:
1) Rhizobium strain testing, in which 23
strains of varying effectiveness were inoculated onto P. erosus
grown in sterile, nitrogen free media.
Included were treatments receiving chemical nitrogen and no Rhizobium
applied. Across this gradient of
symbiotic effectiveness dry weights, components of yield and nitrogen contents
were compared.
2) Diurnal profiles in rates of acetylene reduction
(symbiotic nitrogenase activity) for P. erosus at different
stages of root tuberization.
3) Seasonal profiles on partitioning of dry
matter and nitrogen between plant organs, weekly rates of acetylene reduction,
and the effects of pod removal as a sink manipulation promoting root
tuberization.
Pachyrhizus erosus is one of
very few storage organ crops that are capable of symbiotic nitrogen
fixation. Assimilate stored in the
tuberous root may support nitrogen fixation, while at the same time nitrogen
relations and symbiosis may affect root tuberization. If the extent of diurnal fluctuation in nitrogenase activity is
not altered by increased root tuberization then the pattern of nitrogenase
activity of tuberous-rooted legumes is no different than that reported for
nodulated legumes with fibrous roots.
It is the intent of this thesis to describe the potential for root
tuberization and nitrogen fixation by P. erosus.
CHAPTER
II
LITERATURE
REVIEW
Pachyrhizus erosus - Tropical Root Crop
Pachyrhizus erosus (L.)
(Mexican yam bean) is one of few leguminous root crops. A hairy, twining herb native to Mexico and
Central America, P. erosus is also cultivated in S.E. Asia
(Purseglove, 1968), China, India (Deshaprabhu, 1966), and Hawaii. The lobed, turnip-shaped tuberous root is
perennial, but P. erosus is generally cropped as an annual since
the tuberous roots become fibrous with age.
The root may be eaten raw, is mildly sweet and very crispy. After eating a sliced section some people
unfamiliar with the “chop suey yam” might think this a fruit rather than a
root. It is often used as a substitute
for the Chinese water chestnut in oriental cooking. In 1973, Kay estimated the annual importation from Mexico to the
United States to be 400 tons.
Tropical root and tuber
crops, owing to their high bulk and relatively low value, tend not to be
international trade items (Leslie, 1967).
Even within tropical countries, root crops contribute much less to
agricultural production than the acreage would otherwise indicate because root
crops are often grown as a subsistence food and are not marketed. Root and tuber crops tend to be regarded as
inferior foods, while cereals are often equated with civilization and progress. The motto of the United Nations Food and
Agriculture Organization is “‘Fiat Panis’ - let there be bread” (Coursey and
Haynes, 1970).
Because root crops tend
to be high in carbohydrates and low in protein, vitamins and fats (Leslie,
1967), this bias is not entirely unjustified.
The carbohydrate and protein content of P. erosus is even
lower than that of yam, taro and sweet potato (Ezumah, 1970). Thus the roots from P. erosus
would be a poor major staple for humans.
Additional constraints
against expanding production of P. erosus in the tropics are the
same as for other root crops. The scale
of production tends to be quite small (Ezumah, 1970) and it is manually
harvested (Bautista and Cadiz, 1967; Kay, 1973). Mechanical systems of planting and harvesting root crops have
been developed (Jeffers, 1976) but due to the low value and small scale of
production, initial inputs for increased production should be toward varietal
improvement and expanded use of chemical fertilizers (Johnson, 1967).
Production of P. erosus
by small farmers is encouraged by several cultural attributes of this
crop. It is adapted to the very humid,
hot tropics (Rachie and Roberts, 1974), although short term drought resistance
is provided by the tuberous root.
Insect and disease problems are infrequent (Bautista and Cadiz, 1967)
due in part to the rotenone and pachyrhizid content of the shoots (Deshaprabhu,
1966). Tolerances to stress and pests
allow for adequate yields under low input regimes. A practice easily affordable
to small farmers raising P. erosus is that of flower and pod
removal to promote root tuberization.
Various authors report this to be a traditional practice (Deshaprabhu,
1966; Kay, 1973; NAS, in press) yet experimental results describing the
consequences of depodding are not available.
The young pods may be eaten after thorough boiling (Brucher, 1976).
Appendix (1) lists the
average per acre yield, time to harvest, average price and gross return per
acre for many root crops produced in Hawaii.
No figures were available for P. erosus in the Statistics
of Hawaiian Agriculture for 1977, although 11 other root and
tuber crops were therein reported. When
available in Hawaii, P. erosus retails for more than $.75 per
pound. Assuming current price levels
and a potential for export, P. erosus could offer gross returns
comparable to alternative root crops in Hawaii.
P. erosus is typical
of the major tropical root crops in that it is a nodulated legume, receiving
benefit of nitrogen fixing Rhizobium bacteria (Figures 1a and 1b). Presently little is known about the Rhizobium
requirement or the potential of P. erosus to supply its nitrogen
needs through symbiosis in the field (Marcarian, 1978). The role of legumes in farm ecology goes
beyond directly providing nutrition or profit to producers. Through root nodule symbiosis, legumes act
to restore and maintain the nitrogen status of the soil. The aerial portion of P. erosus
contains much of the total plant nitrogen, and if reincorporated into the
soil, would certainly prove of residual value.
Unfortunately, the shoots
of P. erosus are poisonous and unusable as feed to ruminant
animals. Deshaprabhu (1966) believes
that horses accept this as a forage more readily than do cattle. He also noted that old and non-marketable
roots are useful as fodder. The
poisonous seeds of P. erosus are used as insecticides and fish
poisons. The stems are said to render a
fiber used in Fiji to make fish nets (Deshaprabhu, 1966). Despite the undesirability of P. erosus
residue as animal food, this crop’s acceptance as a food, the potential for
export to temperate areas, the ability to fix atmospheric nitrogen, and the
supplemental uses of non-marketable plant parts allow this crop to be
considered as having under-exploited potential in the tropics.
Productivity and
Partitioning of
Carbohydrates in Root and
Tuber Crops
Solar radiation levels
determine the rate of dry matter accumulation in plants when other conditions
are not limiting. Consequently, time to
establishment of a full canopy after planting determines crop productivity (Loomis
and Rapoport, 1976). Haynes et al.
(1967) have well correlated the leaf area index and yield for several cultivars
of yam (Dioscorea alata (L.)).
The authors felt this is particularly significant since leaf area is
alterable through management practices such as plant spacing, support,
irrigation and fertilization. Net
assimilation rate was also well correlated with storage organ yield during
early stages of growth in yam; however, at later stages of
|
|
storage organ development,
the immediate source of dry matter entering the tuber changes from strictly
recent assimilate to plant translocate from the shoots (Degras, 1967). This is the onset of the “death by
exhaustion” of the aerial parts described by Milthorpe (1967).
By necessity net
productivity does influence yields, but the partitioning of assimilates between
respiration, growth and storage result in an additional feature, unique to root
and tuber crops (Loomis and Rapoport, 1976).
The extent of root sink strength during the final stages of plant life
greatly influences final yield in sugar beet (Beta vulgaris (L.))
(Das Gupta, 1969), potato (Solanum tuberosum (L.)) and Dahlia
sp. (Loomis and Rapoport, 1976).
It is not known if storage organs release growth inhibitors that act to
mobilize substrate to that organ during late stages of growth (Loomis &
Rapoport, 1976).
There are two basic
patterns of storage organ accumulation, 1) balanced and 2) phasic
partitioning. Balanced partitioning as
represented in the sugar beet (Beta vulgaris) is relatively
insensitive to the environment.
Concentric cambia are formed early in ontogeny, roots and shoots develop
synchronously (Mithorpe, 1967). In
phasic partitioning rapid shoot and fibrous root growth precede storage organ
initiation. Tuberization may be triggered by some aspect of the environment,
followed by rapid predominance of the storage organ as a depository for
assimilate (Loomis and Rapoport, 1976).
Short days are known to regulate secondary thickening of roots in
scarlet runner bean (Phaseolus coccineus (L.)), yam (D. alata),
Jerusalem artichoke (Helianthus tuberous (L.)) (Garner and
Allard, 1923) and winged bean Psophocarpus tetragonolobus (L.)
DC) (Lawhead, 1978). Pachyrhizus
erosus (L.) did not tuberize under a 14 hour photoperiod (Bautista and
Cadiz, 1967), while other authors speculate that initiation of tuberous-root
“bulking” in P. erosus is regulated through the photoperiod
(Ezumah, 1979; Marcarian, 1978).
Torrey (1976) stressed
the need of studies concerning hormone flow from the shoot to the root under
different daylengths since the presence of cytokinin has been related to early
secondary thickening of roots. Trapping
of Golgi vesicles by microtubules along the primary xylem has been shown to be
an early state in the secondary root thickening of alfalfa (Medicago sativa
(L.)) (Maitra and Deepesh, 1971).
In conclusion, both external and internal
factors are involved in plant growth and partitioning of assimilate into
storage organs. Exact evidence of these
factors for Pachyrhizus erosus is not currently available except
an indication of a photoperiodic requirement for secondary root thickening.
The Acetylene/Ethylene Assay
of Nitrogenase Activity
The acetylene reduction assay of nitrogen
fixation has been shown to be sensitive, universal, and relatively simple
(Hardy et al., 1968). Nitrogenase, the
enzyme that reduces atmospheric nitrogen also reduces acetylene to ethylene,
cyanide to methane and ammonia, N20 to N2 and water; to
mention a few reactions. Using
acetylene as a substrate for reduction results in sensitivity since only two
electrons are required for each ethylene molecule produced while atmospheric
dinitrogen requires 6 electrons for complete reduction.
The acetylene
reduction technique was shown reliable for free living nitrogen fixing
organisms, as well as with the root nodule symbiosis. Acetylene reduction, as measured by gas chromatography, is a less
time-consuming technique than Kjeldahl analysis or 15N assayed by
mass spectrometry.
Bergersen (1970) compared rates of acetylene
reduction and 15N uptake of soybeans in nitrogen-free media. The ratio of acetylene reduced to nitrogen
fixed (C2H4:NH3) ranged from 2.7 to 4.2. These observations do not invalidate the use
of acetylene reduction to compare nitrogen fixing systems (nitrogenase enzyme
activity); however, this work established
that acetylene reduction is a poor quantitative measurement of exact amounts of
nitrogen fixed.
Mague and Burris (1972)
compared rates of acetylene reduction for intact soybean plants, decapitated
root systems and detached nodules, finding activity ratios of 100/46/23
respectively. Water surfaces on the
root nodules was shown to decrease activity.
Hardy et al. (1973) comprehensively reported on the use of the
acetylene/ethylene assay. It was found
to have been useful in biochemical and physiological studies of the leguminous
and non-leguminous symbiosis, soil, marine, rhizosphere, phylloplane and
mammalian nitrogen fixing systems within five years of its development as a
measurement of nitrogenase activity.
More recently in situ
incubation in acetylene has been used to determine nitrogenase activity. Fishbeck et al (1973) working with soybean
found that if the growth media was sufficiently porous, whole plant incubation
did not result in significant differences from destructive incubation of
nodulated roots. This in situ
technique was used to measure diurnal changes in symbiotic nitrogenase
activity. Since then other authors
(Sinclair et al., 1978) have used the non-destructive acetylene reduction assay
to compare acetylene/N2 reduction rations, as well as plant species
differences in nitrogen fixation.
Periodic in situ assay did not disrupt growth processes of the
many forage species that were compared.
Ruegg and Alston (1978)
used in situ incubation to generate diurnal profiles of nitrogenase
activity for glasshouse grown Medicago truncatula (Gaertn.). Significant diurnal fluctuation was observed
over a two day cycle despite incubation in 10% acetylene for 30 or 60
minutes.
Productivity and Partitioning
in Symbiotic Legumes
Under ideal field conditions light and
temperature levels regulate plant productivity. Wilson et al. (1933) demonstrated that legume growth and
symbiotic nitrogen accumulation were increased as the partial pressure of
carbon dioxide was raised from .03% to 0.8%.
Carbon dioxide is the substrate of photosynthetic productivity, just as
light is the energy source. This
experiment was the first strong indication that assimilate supply to the root
nodules regulate rates of nitrogen fixation and the number, size and
distribution of the root nodules. Later
researchers, comparing 15N accumulation of darkened and illuminated
symbiotic legumes demonstrated the importance of light (and therefore recent
assimilates) on the rate of nitrogen fixation (Lindstrom et al., 1952; Virtanen
et al., 1955). Bach et al (1958)
examined this directly using 14CO2. During the photoperiod 14C accumulated
in the root nodules at twice the rate than at night. This work demonstrated the need of continued supply of
photosynthate to the nodules to maintain maximum rates of nitrogen
fixation. Lawrie and Wheeler (1973)
correlated the rate of acetylene reduction with levels of labelled
photosynthate in pea (Pisum sativum (L.)). The main sink within the nodules for
assimilate was the bacteroidal areas.
Later work by the same authors (Lawrie and Wheeler, 1975) with Vicia
faba (L.) detected 14C in the root nodules within 30
minutes of feeding the shoots 14CO2. Ching et al (1975) related the decrease in
ATP, sucrose, ATP/ADP ratio and nitrogenase activity to prolonged darkness for
1 day using 25 day old soybean. The
energy balance of the nodules was dependent
upon arrival of recent photosynthate.
Nitrogenase enzyme activity
of temperate legumes is not greatly affected by incubation temperatures. Hardy et al (1968) equilibrated and then
incubated nodulated roots of soybean at a range of temperatures. Between 20o and 30o C
there was no temperature effect on acetylene reduction, but a steady decrease
was observed when root temperatures declined below 20o C.
Temperature strongly
affects the supply of carbohydrates to the root nodules. Michin and Pate (1974) using pea (Pisum
sativum) found that higher night temperatures resulted in a more
pronounced decrease in N2 fixation during the night. The authors speculated that nodule
metabolism can utilize limited supplies of carbohydrate more efficiently for
nitrogen fixation at lowered night temperature, since low night temperature
reduces the rate of respiration more than the rate of nitrogen fixation. In the same study respiratory output was well
correlated with nodule soluble carbohydrate.
Reports that changes in
the rate of nitrogen fixation are more strongly correlated with air
temperatures than with soil temperatures implies that temperature plays an
indirect role on nodule function (Mague and Burris, 1972). Sloger et al (1975) found that for field-grown
soybeans soil temperature varied less than nitrogenase activity throughout the
day.
The effect of air
temperatures on the rate of acetylene reduction varies between hosts and Rhizobium
strains. Mes (1959) found that
increasing day temperatures from approximately 20oC to either 25o
or 27oC decreased nitrogen accumulation in the temperate legumes, Vicia
sativa (L.) and Pisum sativum (L.). On the other hand, lowering day temperatures
of tropical legumes, Arachis hypogaea and Stizolobium deeringianum
Bort. depressed nitrogen accumulation.
Similarly, Pate (1962) found that the symbiosis of Medicago
tribuloides (Desr.) was more tolerant of higher temperatures, and that Vicia
atropurpurea (Desf.) was more tolerant to lowered temperatures when the two
species were compared. In general the
symbiosis of tropical legumes are less sensitive to higher temperature regimes
(27o-35oC) than are the temperate legumes.
Physiological Rhythms in
Symbiotic Activity
Using a split shoot
technique with Lupinus augustifolius (L.) in which one of the
shoots was fed 14CO2 and the other shoot was removed for
collection of exudate, Greig, Pate and Wallace (1962) studied fluctuations in
the amino content and radioactivity of the decapitated stem bleeding sap. The diurnal rhythm of temperature stimulated
movement of labeled carbohydrate from the shoots. Specific activity of the amino fraction increased over several
days, indicating continued radio labeled carbohydrate supply to the nodules
after assimilation of 14CO2. Plants maintained in constant temperature and darkness declined
in 14CO2 specific activity over time, translocation of
carbohydrates from the shoot could not offset the depletion of root
reserves. In this way both fluctuations
of temperature and exposure to light were shown to stimulate nitrogen fixation.
Output of cations and
amino compounds in the bleeding sap of nodulated Pisum arverense
exhibited a diurnal rhythm with a maximum near noon and a minimum near
midnight. Labeled amino acids were
recovered after one hour of photosynthesis in 14CO2
(Greig et al, 1962).
An endogenous component
for rhythmic discharge of amino compounds was demonstrated for Lupinus augustifolius
(L.) and Pisum arverense (Pate and Greig, 1964). This occurred for plants under normal light
and prolonged darkness. The amplitude
of the rhythm was increased by cold nights and warm days, which acted to time
this rhythm.
Examination of the
ultrastructure and functioning of the transport system to and from root
nodules of Pisum arverense and Trifolium repens
(L.) (Pate et al, 1969) indicated that normal source-sink processes are
maintained with assimilate supply to the nodules, but that amino acid export
from the nodules was associated with active processes. Ultrastructural studies could not clearly
define the export mechanism.
The
differences in nitrogen fixation between fluctuating temperature/humidity
regimes and constant temperature/humidity conditions were described by Minchin
and Pate (1974) for P. sativum. Acetylene
reduction, root respiration and nodule sugars increased during the photoperiod,
while nodule soluble nitrogen decreased.
The fluctuating environment stimulated overall growth and nitrogen
fixation when compared to constant temperature/humidity. This was due in part to greater rates of
nitrogen fixation under cooler night temperatures, resulting in less
respiration during the dark period.
This study included use of the acetylene reduction assay of nitrogenase
activity. When these results were
compared to bleeding sap estimates of the rate of nitrogen fixation, the
results were in conflict. Bleeding sap
flux greatly overestimated the extent of diurnal changes in nitrogen fixation
because the products of nitrogen fixation were retained during the night, and
not released until plants were rapidly transpiring during the next
photoperiod. In this same study, more
nitrogen was fixed during the night in the fluctuating temperature environment
of 18oC day, 12oC night than during the photoperiod. The authors were not certain whether this is
an artifact of growth cabinet conditions or if this applies to plants growing
in some natural environments.
Examples of Diurnal
Changes
in Nitrogenase Activity
In most cases where
diurnal fluctuation of nitrogenase activity has been observed, the maxima
occurs near the period of maximum light intensity (Hardy et al., 1968). This has been demonstrated in the
non-legumes Alnus Glutinosa and Myrica gale (Wheeler,
1969), and Casuarina sp. (Bond and Mackintosh, 1975) as well as
for quite a few legumes. Nitrogenase
activity of field grown soybeans (Figure 2) consistently showed diurnal
changes; however, the extent of these changes varied between two and three-fold
(Sloger et al, 1975; Hardy et al, 1968) to five-fold (Mague and Burris,
1972). One published report (Ayanaba
and Lawson, 1977) claims to have found no diurnal trend in the field, but when
their results are plotted with other authors a trend does become evident. Some greenhouse (Fishbeck et al, 1973) and
growth chamber (Mederski and Streeter, 1977) were compared to bleeding sap
estimates of the rate of nitrogen fixation, the results were in conflict. Bleeding sap flux greatly overestimated the
extent of diurnal changes in nitrogen fixation because the products of nitrogen
fixation were retained during the night, and not released until plants were
rapidly transpiring during the next photoperiod. In this same study, more nitrogen was fixed during the night in
the fluctuating temperature environment of 18oC day, 12oC
night than during the photoperiod. The
authors were not certain whether this is an artifact of growth cabinet
conditions or if this applies to plants growing in some natural environments.
Examples of Diurnal
Changes
in Nitrogenase Activity
In most cases where
diurnal fluctuation of nitrogenase activity has been observed, the maxima
occurs near the period of maximum light intensity (Hardy et al., 1968). This has been demonstrated in the
non-legumes Alnus Glutinosa and Myrica gale (Wheeler,
1969), and Casuarina sp. (Bond and Mackintosh, 1975) as well as
for quite a few legumes. Nitrogenase
activity of field-grown soybeans (Figure 2) consistently showed diurnal
changes; however, the extent of these changes varied between two and three-fold
(Sloger et al, 1975; Hardy et al, 1968) to five-fold (Mague and Burris,
|
|
1972). One published report (Ayanaba and Lawson,
1977) claims to have found no diurnal trend in the field, but when their
results are plotted with other authors a trend does become evident. Some greenhouse (Fishbeck et al, 1973) and
growth chamber (Mederski and Streeter, 1977) investigations with soybean
suggested considerably reduced diurnal changes with the maxima occurring
nearing the end of the light period.
Descriptions of diurnal
variation in acetylene reduction of field-grown Lupinus luteus
(L.) by different authors are in conflict (Figure 3). Vegetative lupins had no
significant differences in diurnal nitrogenase activity with no pronounced
increase during the photoperiod (Trinick et al., 1976). The same field-grown species, sampled at the
late bud stage by a different investigator (Shaposnikov, 1975) showed about a
fifty-fold difference between the maxima and the minima. These tremendously different findings are
hard to reconcile, despite differences in incubation techniques.
Growth room studies on P.
sativum (Figure 4) indicated a less than two-fold difference between
maximum and minimun nitrogenase activities. Again the maximum activity
occurred during the end of the light period, or into the early dark period
(Michin and Pate, 1974; Lawrie and Wheeler, 1973). Soluble carbohydrate levels in the nodules correlated well with
changes in acetylene reduction (Michin and Pate, 1974). Prolonged darkness for 24 hours resulted in
almost negligible nitrogenase activity.
Longer periods of prolonged darkness also resulted in greater reduction of
nitrogenase activity following reinitiation of the photoperiod (Lawrie and
Wheeler, 1973). Lawrie and Wheeler
(1976) later stated that peak activity often occurs at night.
Field-grown peanuts
(Balandreau et al, 1974) displayed a strongly bimodal curve which the author
concluded to be a product of climatic stress since the minima occurred at
noon. Two cowpea cultivars (Ayanaba and
|
|
Lawson, 1977) also showed
two peaks in acetylene reduction activity during the course of the day, despite
the unimodal nature of temperature and light levels. The daytime nitrogenase peak tended to be much larger than the
dark period peak. In the same
investigation, cowpea variety TVu 1190 sampled at eight weeks showed a
four-fold difference between maximum and minimum activities. The trend in nitrogenase activity was
unimodal with a maxima near noon.
All of the previous
examples of diurnal changes in nitrogenase activity deal with annuals. It is possible that some perennials with
different assimilate storage organs (e.g., tuberous roots) and which
lack strictly determinate
reproductive sinks, could display very attenuated diurnal patterns.
Source-Sink Manipulations
in Legumes
That carbohydrate supply
regulates rates of N2 fixation is supported by observed changes in
nitrogen fixation following photosynthetic source-sink manipulations. Pod removal of soybeans resulted in
increased nodulation and root weight (Loong and Lenz, 1974) indicating that
more carbohydrates reached the root system.
Total plant weight was increased by 70% and 100% pod removal. Lawn and Brun (1974) established a range of
source-sink ratios by depodding, defoliating, shading and providing
supplementary light to soybean.
Treatments designed to enhance carbohydrate supply to the nodules
increased the rates of acetylene reduction and numbers of nodules. Treatments that limited carbohydrate supply
reduced N2 fixation and nodule numbers. The authors speculated that the decrease in N2
fixation during podfill was related to competition for carbohydrates from the
developing pods. Mondal et al. (1978)
showed that removal of pods decreased photosynthetic rates slightly, and that
starch accumulated in leaves as a result of pod removal. Starch accumulation in leaf tissues is
thought to shade chloroplasts, thereby lowering photosynthesis. Pod removal did not prevent a dramatic
decrease in photosynthetic efficiency of leaves about 40 days after flowering
despite the leaves remaining green.
Plant weights or nitrogen fixation were not reported in this study.
Ciha and Brun (1978)
found that depodding resulted in lowered rates of dry matter accumulation, but
total plant weights were similar because of increased leaf duration in depodded
plants. Depodding resulted in an increase
of nonstructural carbohydrates in the leaves and petioles, primarily due to
starch accumulation.
Continuous flower removal
in pea (P. sativum) resulted in an increase in total plant
acetylene reduction, nodule specific activity and total nodule weight (Lawrie
and Wheeler, 1974). Similar results
were obtained by Bethlenflavay et al. (1978); depodding of pea (P. sativum)
increased rates of acetylene reduction, nodule mass and total plant nitrogen
when plants were harvested after 60 days.
Leaf removal decreased the previously mentioned parameters.
In conclusion,
photosynthetic source sink manipulations designed to increase carbohydrate
supply to the roots consistently increase rates of symbiotic nitrogen
fixation. Total plant production does
not necessarily reflect this increase in nitrogen fixation because sink
capability becomes limiting as increased nitrogen fixation and vegetative vigor
are not completely substitutable sinks compared to podfill. Hormonal imbalances resulting from pod
removal, and consequent changes in plant metabolism and morphology complicate
interpretation of these research findings.
Also, many authors do not report changes in root weight as a result of
depodding. Both of these species that
have been described are temperate annuals.
Different plant responses
to depodding could be expected among tropical perennials. Three perennial Desmodium spp.
did not show any relationship between the development of reproductive
structures and root nodules (Whiteman, 1970).
The effects of pod removal and partial defoliation on root tuberization have
been described for Psophorcarpus tetragonolobus (Bala and
Stephenson, 1978; Herath and Fernandex, 1978).
Bala and Stephenson (1978) found no significant differences in tuberous
root weight after 15 weeks of plant growth and seven weeks of periodic flower
removal. After 20 weeks there was an
approximate six-fold increase in tuberous root dry weight in response to
deflowering. Herath and Fernandez
(1978) compared the effects of flower and young pod removal and of vegetative
pruning on four lines of P. tetragonolobus. After five months of growth, flower and
young pod removal had increased the dry weight of tuberous roots three-fold
while vegetative priming had slightly decreased root weight when compared to
the control.
Rhizobium Strain Requirements
Establishing effective Rhizobium
strains for P. erosus has received very little attention. Early studies on effective cross inoculation
groupings within the “cowpea miscellany” did not include Pachyrhizus spp.
hosts, nor were host isolates included among the Rhizobium strains
evaluated (Burrill and Hansen, 1917; Walker, 1928; Allen and Allen, 1939). As the study of the legume symbiosis and
rhizobiology became more diversified, Pachyrhizus spp. remained
overlooked as a nodulated legume.
Currently the Nitragin Company, commercial inoculant producers, markets
rhizobia for P. erosus.
These cultures were obtained in Thailand, and have not been extensively
compared to host isolates from the area of origin, Southern Mexico (J. C.
Burton, personal communication).
Recently Marcarian (1978)
has identified P. erosus as an economic plant well adapted to
stress conditions of the humid, lowland tropics. She has determined this crop’s potential to provide nitrogen
through symbiosis in the field as a current research need. Before this can be done, highly effective
isolates for P. erosus must be identified.
Root nodules similar to
those formed on P. erosus were described by Spratt (1919) as the
Viceae type nodule. It is elongated with
a well defined apical meristem. The
nodule branches and may form very large clusters as with Vicia faba
(L.) and Stizolobium sp. The
bacteroidal zone remains continuous as the nodule develops (Figure 1b) as
opposed to bacteroidal zones which separate into distinct areas adjacent to
vascular tissue.
Establishing known
effective Rhizobium strains for a legume host is an essential beginning
to further studies, including the host’s symbiotic potential in the field. Exploratory tests of this nature should be Rhizobium
strain intensive, particularly if the cross inoculation grouping of a host is
unknown, and if host root nodules or site soils are not available (Burton,
1977).
CHAPTER
III
THE RHIZOBIUM
AFFINITIES OF
PACHYRHIZUS EROSUS (L.)
INTRODUCTION
Pachyrhizus erosus (Mexican
yam bean) is a tuberous-rooted legume which has been identified as a plant
adapted to hot, wet tropical stress conditions (Rachie and Roberts, 1974;
Marcarian, 1978), and is considered a legume of under-exploited potential by
the National Academy of Science (in press).
Although it has low nutritive qualities compared to other root and tuber
crops (Ezumah, 1970; Evans et al, 1977), it is appreciated for its crispness
and mild sweetness when eaten raw. When
cooked, it may be considered a substitute for the chinese water chestnut (Kay,
1973). Although it is presently
exported from Mexico to the U.S. (Kay, 1973) there is much potential to develop
improved varieties and cultural systems.
Published accounts of
Mexican yam bean culture (Bautista and Cadiz, 1967; Kay, 1973) recommend
application of nitrogenous fertilizers and fail to mention that this is a
nodulated legume. Inoculated P. erosus
grown at Paia, Maui (NifTAL Project site) in the field without application of
chemical nitrogen yielded 27 metric tons/ha within 15 weeks (Chapter V). This
is comparable to most other tropical root and tuber crop yields even under
moderate levels of nitrogen fertilization.
Marcarian (1978) suggests that the potential of this crop to provide its
nitrogen requirement through the root nodule symbiosis is a basic research
need.
Before field comparisons
of yields from inoculated and nitrogen fertilized legumes should be conducted,
the Rhizobium strain requirement of a given legume must be
evaluated. The purpose of this research
was to identify effective Rhizobium strains for P. erosus,
to draw inferences concerning the effective cross inoculation group to which
this species belongs, and to compare the growth and nitrogen contents of
symbiotic and nitrogen fertilized P. erosus.
MATERIALS AND METHODS
The technique of
establishing legumes in a sterile, nitrogen free media inoculated with various
strains of Rhizobium makes it possible to rank strains by
effectiveness. One liter “Leonard jar”
assemblies (Vincent, 1970) were employed using a vermiculite filled upper
container which was connected by a cotton wick to a two liter reservoir filled
with full strength Broughton and Dilworth solution (1971). These assemblies were sterilized by
autoclaving at 121oC and 15 psi for 45 minutes.
Seeds of P. erosus
(Tpe-1 from IITA) were treated in concentrated sulfuric acid for five minutes,
then repeatedly rinsed in sterilized water. These were germinated on to water
agar, selected for uniformity, planted two per vessel and inoculated with two
ml. of a turbid suspension of the intended rhizobia (= 2 x 109
rhizobia/ml). Twenty three strains from
the NifTAL culture collection were compared in this fashion (Table 1) after
being raised in yeast extract-Mannitol broth (Vincent, 1970). Two uninoculated controls (zero N and 70 ppm
N - supplied as KNO3) were included. The “Leonard jars” were placed in the glasshouse in a randomized
complete block design with 25 treatments replicated three times. After 60 days all treatments were harvested
and nodule observations taken. Shoots
and roots were separated and oven dried.
Selected treatments were analyzed for total nitrogen by a colorimetric
technique (Mitchell, 1972).
RESULTS AND DISCUSSION
The dry matter yield,
percentage nitrogen in tissues and total nitrogen content of the roots and
shoots revealed a wide range of symbiotic effectiveness for the Rhizobium
strains tested (Table 1, Table 2, Figure 5, and Appendix 2). The most vigorous of the symbiotic
treatments did not produce as much dry matter as the nitrogen-supplied control,
but assimilated more total nitrogen.
The percentage nitrogen in the tuberous roots of the control treatments
(zero nitrogen and 70 ppm N) was lower than in most of the symbiotic treatments
(Table 2 and Appendix 2).
The proportion of total
dry matter in the tuberous root was not influenced by nitrogen source or
symbiotic effectiveness (Tables 2 and 4, Appendix 2). Long dark periods promote secondary thickening of P. erosus
(Bautista and Cadiz, 1967; Kay, 1973) and other legumes (Garner and Allard,
1923). Since these plants were grown
during short days, it is assumed that partitioning of assimilate was a
photoperiodic effect and, therefore, independent of nitrogen nutrition over the
ranges tested. However, the proportion
of total plant nitrogen in the tuberous
root was related to the symbiotic effectiveness of the Rhizobium
strain. This is not surprising since
nitrogen availability was limiting plant growth. Nitrogen storage in the tuberous roots depended on the nitrogen
status of the plant as a whole (Tables 2 and 4).
Certain Rhizobium strains associated
with legumes common to the natural habitat of P. erosus varied in
their ability to nodulate and fix nitrogen. Isolates from Phaseolus vulgaris
(L.) and Leucaena leucocephala (L.) did not nodulate P. erosus. A R. lupini strain (Tal
1102) established a partially effective symbiosis resulting in low tissue
nitrogen concentrations, but relatively high dry matter accumulation. Tal 22 and Tal 731, belonging to the Phaseolus
lunatus-Canavalia subgrouping of the “cowpea miscellany” were
only partially effective.
Two Rhizobium strains widely used in
commercial inoculum for many
|
|
|
|
|
|
tropical legumes belonging to the broad
“cowpea miscellany”, Tal 309 (CB756) and Tal 169 (Nit 176A22) were also only
partially effective. The percent
nitrogen in the plant tissues was high, but total dry matter and nitrogen
accumulation was less than 50% of that obtained with the best strains. Many small, ineffective nodules resulted
from inoculation with Tal 742, an isolate from Desmodium heterophyllum
DC., a widely distributed Desmodium sp. with a reputation of specificity. The nitrogen demand of this ineffective
nodule sink resulted in very low concentrations of nitrogen in the tuberous
roots (0.28%).
The effectiveness of host
isolates-from Pachyrhizus sp. was quite variable. Tal 656 produced nodules that were
completely ineffective while Tal 657 was among the better strains. Both of these strains were collected from
the same site in Malaysia. Thus it
appears likely that ineffective nodulation must frequently occur in the field.
The most effective strain
was a fast growing isolate from Crotalaria juncea (L.). Walker (1928) classified this species as
belonging to a separate cross inoculation group from the broad “cowpea
miscellany” and other Crotolaria spp. Allen and Allen (1939) reported that C. juncea and C.
spectablis Roth. were nodulated by a wide range of “cowpea type” rhizobia,
but did not report the effectiveness of the nodules formed. The fact that a strain from C. juncea
was the most effective among the diverse strains tested deserves the attention
of additional studies to determine whether or not P. erosus and C.
juncea belong to the same effective cross inoculation group.
Nodulation seldom
occurred on the taproot of P. erosus, rather the early secondary
roots were nodulated. Effective nodules
were elongate and branching. The active
bacteroidal region of the nodules was continuous (Viceae type, after Spratt,
1919), and migrated as the nodule elongated (Figure 1b) with the oldest region
of the nodule interior turning green, but not decomposing with age. Based on the size and longevity of root
nodules observed in lengthier pot studies and in the field, the nodules of P.
erosus may be functionally perennial.
However, the earliest nodules to form on the root system are spacially
displaced and crushed or are severed from the roots as the storage organ
expands (see Figure 15).
Total plant nitrogen was
highly correlated with plant dry weight and also with tuberous root nitrogen
(Table 3). Tissue nitrogen
concentrations of the shoots and roots were significantly correlated with
symbiotic effectiveness, but at a lower level of confidence. This is in agreement with the findings of
Duhigg et al (1978) when individuals of a single alfalfa cultivar (Medicago
sativa (L.) cv. “Mesilla”) were compared for their ability to fix
nitrogen.
As was mentioned
previously, various authors (Bautista and Cadiz, 1967; Ezumah, 1970) have
speculated that root tuberization of P. erosus is regulated by
the photoperiod, as it is with Phaseolus coccineus (L.) (Garner
and Allard, 1923). Our data seems to
support this speculation since the proportion of total dry matter partitioned
into the tuberous root was constant irrespective of plant nitrogen nutrition
(Table 4). The low levels of nitrogen
in the tuberous root of the nitrate supplied treatment suggests that nitrate
reduction occurs largely in the shoots, and that the reduced nitrogen is not
readily partitioned into the tuberous root.
The extent of nitrogen accumulation in the tuberous root may therefore
be related to the form in which the nitrogen is supplied to the plant. The fact that some Rhizobium strains
(i.e., Tal 309, Tal 656) which have low symbiotic effectiveness resulted in
high nitrogen concentrations in the tuberous root support this observation.
|
|
|
|
SUMMARY
The National Academy of Science called
attention to the Mexican yam bean (P. erosus) as an
“under-exploited” legume. Recommendations
for cultivation of this tuberous root crop include fertilization with nitrogen,
suggesting ignorance of, or inadequacy of, the nitrogen contribution from this
legume’s association with Rhizobium.
Twenty-three strains of Rhizobium of widely differing origins
were used to inoculate P. erosus (Tpe-1 from IITA, Nigeria). Growth of inoculated P. erosus
plants in Leonard jar culture was compared to uninoculated plants receiving no
combined nitrogen and uninoculated plants receiving combined nitrogen (70 ppm N
as KNO3) in the rooting medium.
P. erosus was nodulated by 20 out of the 23 strains of Rhizobium
but formed highly effective symbiotic associations with only two strains. The best strains had been isolated
originally from Crotolaria juncea and Calopogonium caeruleum. Strains from Arachis hypogaea
and Pachyrhizus tuberous also proved moderately effective. The results suggest that there is a
potential to increase field performance of P. erosus through
inoculation with superior strains of Rhizobium at the time of
sowing. The best strain (TAL 734)
produced 80% of the dry matter observed in the combined nitrogen treatment. Partitioning of dry matter between the root
and shoot was not affected by strain of Rhizobium nor source of nitrogen
(symbiotic vs combined). The most
effective strain increased the nitrogen content of the tuberous root three-fold
over the uninoculated control and in the case of an ineffective strain (TAL 742)
the nitrogen content was actually below that of the control (0.28% vs 0.52%
N).
CHAPTER
IV
DIURNAL CHANGES IN
SYMBIOTIC NITROGENASE
ACTIVITY OF THE
TUBEROUS-ROOTED LEGUMES
PACHYRHIZUS EROSUS (L.) AND
PSOPHOCARPUS TETRAGONOLOBUS
(L.) DC.
INTRODUCTION
Under field conditions,
symbiotic nitrogenase activity as measured by the acetylene reduction technique
fluctuates diurnally. This has been
observed in Glycine max (L.) Merr. (Hardy et al., 1968; Mague and
Burris, 1972; Sloger et al., 1975; Ayanaba and Lawson, 1977), Arachis hypogaea (L.)
(Balandreau et al., 1974) and Vigna unguiculata (L.) Walp.
(Ayanaba and Lawson, 1977). Glasshouse
studies indicate this is also the case in non-leguminous symbiosis (Wheeler,
1969; Bond and Mackintosh, 1975) as well as in the rhizosphere of field grown
rice (Oryza sativa) (Balandreau et al., 1974).
Fluctuations in symbiotic
nitrogenase activity observed in the field result from changes in light
intensity and temperature (Mague and Burris, 1972). The former regulates photosynthate supply, the latter affects
basal metabolic rates of photosynthetic utilization for both host and
microsymbiont. Sloger et al. (1975)
found that during cloudy days diurnal fluctuation in the symbiotic nitrogenase
activity of field grown soybean was greatly reduced. Also average specific nitrogenase activity was significantly
correlated with average air temperature but not with average soil temperature,
thus the rate of nitrogen fixation is dependent upon the temperature of the
photosynthetic organs rather than that of the root nodule environment. Bimodal profiles have been accounted to
midday vapor pressure deficit in cowpea (Ayanaba and Lawson, 1977) and to
reduction of atmospheric humidity around peanut (Balandreau et al., 1974). Both of these bimodal observations occurred
in the tropics.
The concept that
carbohydrate supply to the nodules acts as the regulator of nodule activity has
been reviewed by Pate (1976). During
the photoperiod, not only is more nitrogenase activity resulting from increased
photosynthate arriving from the shoot, but nodule soluble carbohydrates and
insoluble starch pools are being replenished (Minchin and Pate, 1974).
Consequently, the magnitude of carbohydrate supply differences between photo
and dark periods is not necessarily reflected in the products of nitrogen
fixation or measured nitrogenase activity.
Minchin and Pate (1974) have demonstrated this in the growth room using
pea (Pisum sativum (L.)) grown in fluctuating day, night
temperatures and humidities. More
nitrogen fixation took place during the dark period than the photoperiod when
temperatures were 12oC and 18oC respectively.
Tuberous-rootedness may
greatly alter carbohydrate supply patterns to the root nodules since shoot
translocates must pass through a taproot with increasing assimilate
demand. At the same time soluble
carbohydrates stored in the tuberous root may be available to the energy demand
of the root nodules at night. Reports
concerning diurnal changes in symbiotic nitrogenase activity of tuberous-rooted
legumes are not found in the literature.
Consequently a glasshouse experiment was conducted to describe the
diurnal patterns in acetylene reduction using Pachyrhizus erosus
(Mexican yam bean) and Psophocarpus tetragonolobus (Winged
bean). Later growthroom, glasshouse,
and field studies sought to elucidate possible relationships between root
tuberization and observed patterns in symbiotic nitrogenase activity using Pachyrhizus
erosus as a host rather than Psophocarpus tetragonolobus
because of the former’s more rapid secondary root thickening.
MATERIALS AND METHODS
Experiment 1. Diurnal pattern of Psophocarpus tetragonolobus
and Pachyrhizus erosus.
Two winged bean lines
(Tpt-1 and Tpt-3) and a Mexican yam bean line (Tpe-1) from the International
Institute of Tropical Agriculture, Ibadan, Nigeria, were tested for acetylene
reduction at varying times of day.
Seeds were surface sterilized in 30% household bleach for six minutes,
rinsed in .01N HCl for 5 minutes followed by five rinses with sterilized
distilled water. Seeds were germinated
on sterile water agar petri dishes.
Two-liter pots were filled with vermiculite, planted with three seeds
per pot and connected to a sterile subirrigation system modified after Weaver
(1975). The nutrient solution used was
a modification of Broughton and Dilworth solution (1971) adjusted to pH 6.8 in
which 2.5% Fe chelate was substituted for the .02M iron citrate stock
solution. After emergence plants were
thinned to one plant per pot and inoculated with 1.0 ml. of yeast mannitol
broth containing appropriate rhizobia (= 109 cells/ml). The pots were arranged in a randomized
complete block design with five replicates.
Thirty-eight days after
planting, plants were sampled for acetylene reduction in a non-destructive
fashion using 20 liter plastic incubation vessels injected with to acetylene
and incubated for one half hour (Figures 6 and 7). Sampling was at selected intervals not less than four hours
apart. Between incubations, plants were removed from the larger vessels and
exposed to moving air. Samples were
stored in 10 ml. vacuum tubes and measured for ethylene production by injection
into a Varian Aerograph gas chromatograph containing a "Poropak-R"
column. Results were expressed as U
moles ethylene produced per plant per hour.
Experiment 2.
Diurnal changes using different plant propagules of
P. erosus.
A second glasshouse
experiment tested the extent of which plants resultant from seeds versus those
propagated from tuberous roots show diurnal fluctuation in symbiotic
nitrogenase activity. Seeds were
treated with concentrated sulfuric acid for five minutes and rinsed several
times in sterile, deionized water.
Fresh tuberous roots weighing approximately 1.2 kg. were selected from
the field, decapitated non-tuberous roots removed, and thoroughly washed. Both propagule types were planted in 20
liter pots containing a mixture of vermiculite and peralite (1:1 v/v). Tuberous roots were planted one per pot,
seeds at two per pot. These pots were
watered daily with 1.0 liter of the nutrient solution previously
described. Upon emergence plants originating
from seed were either inoculated with 1.0 ml. of a turbid suspension of Tal 657
or with 1.0 ml. of that suspension diluted 100-fold with quarter strength
nutrient solution. The plants from
tuberous roots were inoculated with the same 100-fold dilution. Inoculum was diluted to assure that rhizobia
would be well distributed around the exterior of the large tuberous root. The design was a randomized complete block
with five treatments and four replicates.
After 46 days, all plants were sampled for acetylene reduction
destructively by incubation of fibrous root systems in 2.0 liter vessels
injected with 5.0% acetylene. Ethylene
was determined by gas chromatography as previously described at either 0200 or
1400 hours.
Experiment 3. Nitrogenase patterns in field grown P.
erosus.
Seeds of Pachyrhizus
erosus (Tpe-1) were scarified, inoculated with a peat carrier containing
6.3 x 107 rhizobia of strain Tal 657 per seed, and planted in a
randomized complete block design with four replications. Row spacing was 75 cm. with four seeds
planted per meter of row (53,333 plants/ha).
Bagasse at 0.6% dry weight basis had been recently incorporated to
reduce available nitrogen in the soil (a Typic Haplustoll, elevation 100
m.). Basal levels of potassium,
magnesium phosphorus (as treble super phosphate), iron and molybdate were
added. Plants were watered every other
|
|
day prior to emergence
and once weekly thereafter.
Light intensity was
measured as μ einsteins/m2/sec. on a Li-Cor quantum
meter but the measurements do not represent light levels during the exact time
of sampling. Air temperatures were
measured inside the canopy using a shaded bulb thermometer. Soil temperatures were recorded at the 15
cm. depth, temperatures inside the incubation vessels were monitored by
insertion of a thermometer through the rubber septum of an unincubated control
vessel.
Acetylene reduction
activity was determined for four plants one meter of row) in each plot by
incubating root samples for one hour with 5% acetylene in 2.0 liter vessels and
immediately analyzing for ethylene by gas chromatography. Vessels were immediately placed into a
shaded, insulated container equilibrated at 27oC. These were transferred within 25 minutes to
the laboratory where the samples were maintained at 27oC prior to
injection into the gas chromatograph.
Alternate rows were sampled at different stages of root
tuberization. Young, non-tuberous
plants were harvested after three weeks, mildly swollen taprooted plants after
seven weeks and turnip shaped tuberous-rooted plants after twelve weeks. At the later samplings, multiple vessels per
plot were required due to the large size of roots. Vigna unguiculata (cv. California Blackeye) was planted
into rows vacated by the week 3 sampling.
Rates of acetylene reduction were compared at different times of day to
tuberous-rooted P. erosus when both species were at the early pod
stage.
Experiment 4. Effect of prolonged darkness on nitrogenase
level.
P. erosus was grown
from seed in the glasshouse for 15 weeks using the method described in
Experiment 2 in a completely randomized design with three replicates. These plants were then moved into a growth
room with a 12-hour photoperiod of 160 μ
einsteins/m2/sec. of
photosynthetically active radiation.
Constant leaf temperatures were maintained at 31.4± 0.3oC by
evaporative cooling of the air during the day and supplemental heating at
night. After an acclimatization period
of two weeks, plants were destructively sampled for acetylene reduction during
the normal light and dark periods.
Following this, prolonged darkness was initiated and samples were taken
8, 32 and 174 hours into the prolonged darkness period.
RESULTS
Experiment 1.
Nitrogenase levels at
various times during the day are given in Table 5. At the time of sampling, all of the plants had developed tuberous
roots. Total acetylene reduction activity of both tuberous-rooted legume species
increased rapidly during the morning and did not decline until late afternoon
or early evening (Table 5). Air
temperatures were better correlated with nitrogenase activity than were root
temperatures (Table 6). These environmental factors are discussed further under
Experiment 3.
Experiment 2.
The acetylene reduction
activity per unit of nodule weight (nitrogenase specific activity) of plants
propagated from transplanted tuberous roots varied to the same extent as those
started from seed (Table 7). The
concentration of rhizobia in the inoculant broth did not affect nodule mass or
nitrogenase activity of those plants raised from seed when Rhizobium
numbers were held constant (Table 8).
Despite initial shoot dormancy, dry matter increase was greatest in
plants developed from tuberous roots.
Later work indicated that dormancy in tuberous roots of P. erosus
could be overcome by short term acetylene incubation (Appendix 5). The
|
|
|
|
|
|
|
|
first roots to emerge
from the tuberous root were very fleshy, non-branching and were not infected by
rhizobia until they attained several cm. in length.
Experiment 3.
The extent of
fluctuations in specific nitrogenase activity in field grown P. erosus
did not change with different stages of root tuberization (Figure 8 and Table
9). During week twelve, specific
activity levels were reduced compared to earlier observations. After three weeks of growth, nitrogenase
activity was very highly correlated with the solar radiation levels (r=.906*)
and to a lesser extent with air temperature (r=.800) and soil temperature
(r=.776).
Experiment 4.
Symbiotic nitrogenase
activity persisted through 174 hours of prolonged darkness, maintaining a level
equal to 40% of that during the normal dark period (Table 10). This is discussed later in the text. Under
constant temperature and relatively low light levels there were no significant
changes in acetylene reduction between the normal dark period and the
photoperiod, indicating the importance of fluctuating environment on symbiotic
nitrogenase activity.
DISCUSSION
Of the approximately 290
nodulated genera belonging to the Papilionatae, very few have tuberous
roots. It is more likely that tuberous
roots developed on nodulated root systems than vice versa. Just as Lawn and Brun (1974) have shown that
source-sink manipulations affect rates of nitrogen fixation in soybean, root
tuberization would also be
|
|
|
|
|
|
expected to compete with
the root nodules for supply of assimilates.
Yet if nitrogen supply is limiting plant growth, it is possible that
stored carbohydrate from the tuberous root could help satisfy the energy
demands of root nodules, particularly during the dark period when activity is
normally lowered. Another possibility
could be that all of the carbohydrate supply to the root nodules is regulated
through the tuberous root as it develops.
Such regulation of nodule assimilates through the tuberous root could
have the effect of attenuating diurnal changes in nodule activity.
The extent of diurnal
fluctuation in symbiotic nitrogenase activity as affected by root tuberization
has been examined in two ways: a) different propagules (taproot vs. tuberous
root) and b) different stages of tuberous root development over time. Diurnal changes in nitrogenase specific
activity were highly significant but were not altered by the degree of
tuberous-rootedness in either case. The
field grown tuberous-rooted legumes reported here and annual crop legumes have
been shown to be similar in their ratios between maximum and minimum activity
values (Table 11).
The applicability of the
non-destructive method of sampling was confirmed by the fact that greenhouse
grown plants increased in nitrogenase activity between 0900 and 1300 hours by
400, which was identical to the results obtained in Experiment 2 when sampled
destructively (Table 11).
In Experiment 2 the type
of propagule did not affect the ratio of maximum and minumum nitrogenase
activities (Tables 7 and 11) when root nodules of the same age were compared on
plants with very different root systems.
That no root nodules
formed on the first roots to emerge from the tuberous root may be related to
the morphology of the roots which were
|
|
fleshy, lacked fibrous
secondary roots and appeared to serve primarily for plant support. This resistance to early infection may also
be related to the superior nitrogen status of tuberous roots. The average tuberous root propagule
contained more than 1500 mg N (120 g. x 1.3%N dry weight basis) whereas a seed
contained only 9 mg N (0.2 g. x 4.1%N).
Evans et al (1977) have shown that a considerable portion of the
nitrogen in the tuberous root of P. erosus is comprised of free
amino acids and non-amino nitrogenase compounds, and these compounds are
probably available to support new growth of roots and shoots.
The nodules of P. erosus
were elongate and multi-branched. It
was observed that as the nodules aged, a decreased portion of the total nodule
mass was actively bacteroidal tissue.
At the same time the rapidly expanding tuberous root spacially displaced
the nodules and their connective roots.
If this loss of functional nodules were to shift the plant into a less
N-sufficient mode, then this should result in less carbohydrate utilization in
the shoots and additional storage of carbohydrates in the tuberous roots. Selected increments of root nodule
displacement can be said to alter the plant’s nitrogen relations such to
promote continued root tuberization.
No root nodules form on
the upper taproot of P. erosus, rather the earliest nodules occur
on secondary roots. Perhaps
tuberous-rootedness in legumes has been selected as a host countermeasure
against excess nodulation.
Environmental factors
also acted to lower nodule specific activity. Rainfall of 122 mm. was recorded
in the 8 days prior to the final sampling date. Waterlogging is known to disrupt nodule function (Mague and
Burris, 1972; Minchin and Summerfield, 1976).
Soil fauna occasionally attacked root nodules. However, predation upon one section of a multi-branched nodule
did not seriously disrupt other sections of that same nodule.
Both light and air
temperature were well correlated with measured enzyme activity. The multiple
correlation for the equation
Y
= 270 - 0.58 X1 - 5.84 X2 + 2.2 X3
was significant to the
950 level (R = .928) where X1 = air temperature (oC),
X2 = soil temperature (oC), X3 =
photosynthetically active radiation in micro einsteins·m-2·sec-1
and Y = predicted nitrogenase specific activity. Light intensity accounted for most of the
variation in nitrogenase levels (r = .908).
Fitting the observed
diurnal acetylene reduction values from week three to a Fourier periodic curve
(Figure 8) generated the equation
Y
= 102.8 + 24.6 cos (cs) + 14.1 sin (cx)
where x is an observed
time and c is a constant equal to 360o divided by 24, the number of
units in the diurnal cycle. In this
case r = .83 and is significant at the 950 level. The predicted maxima (from θtan) occurs at 1200 hours.
Another interpretation of
the week three diurnal profile is a two phase linear “sawtooth.” Using this model levels remained depressed
or increased very slightly throughout the dark period (r = 0.93, b = 0.67 μ moles
ethylene·g nodules-1 hr-1). With resumption of the photoperiod, nitrogenase levels increased
steadily until the mid-afternoon (r = 0.86, b = 5.94). The two phase linear (“sawtooth”)
interpretation was less significant than either the multiple linear or the
periodic interpretations, owing to loss of degrees of freedom. Also there were no sampling points between
mid-afternoon and early evening, consequently an important phase of the cycle
was not described.
The extent of diurnal
fluctuation in symbiotic nitrogenase activity of field grown P. erosus
at three stages of root tuberization is compared to that of Vigna unguiculata
in Table 12. Attenuation of diurnal
changes in nitrogenase with increased root tuberization would be evident in the
interaction term. This was not the
case; significant changes in activity at different times of day for both
species at all three sampling dates and stages of root tuberization were
observed, and the interaction term was not significant in any of these
situations.
That nitrogenase activity
continued through 174 hours of prolonged darkness is impressive but cannot be
taken as direct evidence of tuberous root support of root nodules. Other investigators (Hardy et al., 1968)
have found that legumes without tuberous roots also continue to fix nitrogen
during prolonged darkness periods.
Investigations directly comparing tuberous-rooted and non-tuberous legume
cultivars’ abilities to fix nitrogen would be facilitated if non-tuberous lines
of Pachyrhizus erosus (L.) were known and available. Other species which could provide these
comparisons would include Vigna unguiculata vs. V. vexillata
(L.) Benth. and different root types of Psophocarpus tetragonolobus
. Although much of the carbohydrate in
the tuberous root of Pachyrhizus erosus is in the form of soluble
sugars and could presumably be mobilized to support symbiotic nitrogen
fixation, these assimilates do not seem to provide any buffering effect for
maintaining nitrogen fixation at a constant rate throughout the day.
The knowledge of daily
nitrogenase activity profiles does have the advantage of providing a better
basis for selecting sampling times of day that result in minimal variance. Diurnal patterns generated in the glasshouse
and field indicate that nitrogenase activity increases rapidly during the
morning and stays relatively constant throughout the later morning and early
afternoon for both of the tuberous-rooted tropical legumes tested. Quantitative description of the fluctuation
in daily nitrogenase activity as measured by acetylene reduction also permits
the extropolation of daily nitrogenase activity from single timepoint
observations. This had been done for
peanut (Balandreau et al., 1974), soybeans (Bezdicek et al.,
|
|
1978) and alfalfa (Duhigg
et al, 1978). The later two authors
included mention of this in their materials and methods, indicating the
importance of diurnal variation in symbiotic nitrogenase activity as a
methodology study.
In conclusion, the sink
capacity of the root nodules as measured by acetylene reduction in
tuberous-rooted, symbiotic legumes appears to be as dependent upon recent
photosynthate as are normal rooted legumes. Sampling for maximum acetylene
reduction activity should be undertaken during the late morning and early
afternoon.
SUMMARY
Two tropical
tuberous-rooted legume species, Pachyrhizus erosus (L.) (Mexican
yam bean) and Psophocarpus tetragonolobus (L.) DC. (winged bean)
fluctuate in their daily nitrogenase levels as measured by acetylene
reduction. Additional investigations
compared the extent of diurnal fluctuation to increased root tuberization. Root tuberization does not alter the daily
nitrogenase profile observed in seedlings of P. erosus. Nodule activity of the Mexican yam bean
continues through 174 hours of prolonged darkness.
CHAPTER
V
ACCUMULATION AND
DISTRIBUTION OF DRY MATTER
AND NITROGEN IN PACHYRHIZUS
EROSUS (L.)
INTRODUCTION
Total plant growth, and
the partitioning of that production into the storage organ are basic factors in
the yield of tuberous roots attainable in Pachyrhizus erosus
(Mexican yam bean). Description of the
patterns of tuberous root growth and nitrogen accumulation over time are not
available for P. erosus, even though this plant has been
identified as adapted to the stress conditions of the humid tropics (Rachie and
Roberts, 1974) and is considered to be a tropical legume of under-exploited
potential by the National Academy of Science (in press).
P. erosus is a
symbiotic legume, yet the use of nitrogenous fertilizers has been recommended
in its culture (Bautista and Cadiz, 1967; Kay, 1974) without mention of
nodulation. Marcarian (1978) stated
that the potential of this crop to fix nitrogen in the field is not currently
known.
Flower and pod removal
have been shown to increase root weight in another tuberous-rooted perennial
legume, Psophocarpus tetragonolobus (L.) DC. (Bala and
Stephenson, 1978; Harath and Fernandez, 1978).
Flower removal is practiced with P. erosus under
traditional cropping systems (Deshaprabhu, 1966; Kay, 1973; NAS in press) but
no detailed description of the response of the plant to this source-sink
manipulation is available.
In this experiment, inoculated P. erosus
was grown without the addition of chemical nitrogen. Plants were harvested at selected intervals and evaluated for 1)
accumulation and partitioning of dry matter and nitrogen, 2) nodulation and
nitrogen fixation, and 3) the effect of periodic flower removal on root
tuberization and plant growth.
MATERIALS AND
METHODS
A field experiment was carried out at the
NifTAL site near Paia, Hawaii on a Typic Haplustoll soil with a pH of
approximately 6.0. Bagasse at 0.6% dry
weight basis was incorporated to reduce available nitrogen in the soil. Basal levels of potassium, phosphorus (as
treble super phosphate), magnesium, iron and molybdate were tilled into the
soil prior to planting.
Seeds of P. erosus (Tpe-1)
originally from the International Institute of Tropical Agriculture, Ibadan,
Nigeria were scarified in concentrated sulfuric acid for five minutes, followed
by repeated rinses with tap water.
These were then inoculated with a peat carrier containing 6 x 107
rhizobia per seed of strain Tal 657 (=UMKL 82, an isolate obtained from the
University of Malaya) and planted in a randomized complete block design with four
replications on August 8, 1978 (Figure 9).
Row spacing was 75 cm with four seeds per meter of row (53,333 plants
per hectare). Plants were watered every day prior to emergence, and once weekly
thereafter.
Plants were harvested at
selected intervals, divided into components, and oven dried at 65oC. Acetylene reduction activity of the root
nodules was determined for four plants (one meter of row) in each plot by
incubating root samples for 1 hour at 27oC with 5.0% acetylene in
2.0 liter vessels and immediately analyzing for ethylene using a Varian
aerograph gas chromatograph containing a “Poropak-R” column. Results were expressed as μ moles
ethylene evolved per plant per hour.
Root nodules were separated from the root and oven dried. Nitrogen
|
|
determinations were made
by digestion in sulfuric acid followed by a colorimetric determination of
ammonium after the technique of Mitchell (1972).
In each block, 5 meter
sections of row were chosen at random for flower removal treatment. All inflorescences were removed each week
from these row sections beginning 10 days after first bud and continuing until
harvest. These plants and comparable
controls were then harvested at 15 weeks and fresh and dry weights and nitrogen
content were determined for shoots, flowers and pods, and tuberous roots. Total dissolved solids from the supernatant
of crushed, centrifuged tuberous roots were measured with an American optical
hand held refractometer.
RESULTS AND DISCUSSION
Dry Matter Accumulation.
The growth of different
plant components over time is given in Figure 10. Rapid shoot growth preceded storage organ accumulation which in
turn preceded inflorescence development and podfill. This pattern of storage organ “bulking” is similar to that of
potato (Solanum tuberosum (L.)), yams (Dioscorea alata
(L.)) and cassava (Manihot esculenta (Crantz.)) described by
Milthorp (1967) and Loomis and Rapoport (1976). P. erosus thus follows the phasic pattern of
storage organ accumulation in which early vegetative growth is characterized
by predominance of shoot and fibrous root growth. Storage organ “bulking”
begins later in the growth cycle of the plant and may require environmental
induction (Loomis and Rapoport, 1976).
For example, P. erosus did not tuberize during 14 hour
photoperiods (Kay, 1973) but tuberized readily in Hawaii, especially under
short days. Phaseolus coccineus
(L.) is another example which is known to have a short day requirement for
initiation of secondary root thickening (Garner and Allard,
|
|
1923). The change to storage organ growth, and
later to podfill, was dramatic. At seed
maturity the aerial portions of P. erosus senesce (Deshaprabhu,
1966). This is the “death by
exhaustion” described by Milthorp (1967) of potato except that instead of
nutrient and assimilate migration to one sink, there are two strongly
competitive sinks: the tuberous roots and the reproductive organs.
Once established, the
reproductive structures and the storage organ competed equally for assimilate
despite the positional advantage of the pods.
P. erosus appears to be unique in that two strong sinks
are in operation simultaneously and are in strict competition with one another,
rather than the situation in which assimilate from the tuberous roots is used
to support seed development as in the case of radish (Raphanus sativus
L.) or sugar beet (Beta vulgaris). The tuberous root of P. erosus is the only
perennial feature of the plant in its natural life cycle. If the tuber is left in the ground, some
carbohydrates and nutrients stored in the tuberous root are eventually used to
re-establish new vegetative shoots after the aerial portion of the plant
dies. Tuberous-rootedness thus
indirectly provides an additional opportunity for seed production the next
season.
Nitrogen Accumulation and
Tissue Concentration.
While the storage organ
and the sexual reproductive sinks may compete equally for carbon (Figure
10), podfill is a stronger sink for nitrogen than is the tuberous root
(Figure 11). Plant nitrogen, whether
symbiotic or absorbed, passes through the tuberous root as it is translocated
upwards in the xylem to the aerial portion of the plant. It may be concluded that even though the
tuberous root has a positional advantage for nitrogen accumulation, it
is at a competitive disadvantage for nitrogen compared to the developing
pods and seeds.
The pattern of nitrogen
accumulation in the vegetative shoot is similar to that of dry matter. There is a phase or rapid nitrogen
accumulation; then a plateau is established, followed by diversion of nitrogen
to the rapidly expanding tuberous root.
Initially, nitrogen accumulation in the reproductive parts lags behind
that of the storage organ, but later nitrogen accumulation is faster in the
developing pods and seeds (figure 11).
After twenty weeks of growth, 205 kg/ha of nitrogen had accumulated in
the crop. Of this, 37%, 23% and 40% were found
in the vegetative shoots, the tuberous roots and the sexual reproductive
structures, respectively.
The phasic pattern of partitioning into the
shoots followed by accumulation of nutrients in the roots was reflected in the
tissue nitrogen concentrations (Figure 12).
The nitrogen concentration in the shoots increased steadily until the
fifth week following emergence. At this
time shoot nitrogen concentrations reached a plateau and nitrogen then began to
accumulate in the roots. When flowering
was initiated, both shoot and root nitrogen concentrations decreased
significantly. The percentage
concentration of nitrogen in the reproductive parts also decreased as the
nitrogen was steadily diluted during peduncle development. At the final harvest much of the plant stem
tissue was associated with the peduncles.
It is important to note that during the
period of storage organ “bulking” (after week 8) the nitrogen concentration of
the root storage organ remained relatively constant. Consequently the time of harvest did not greatly affect the
percentage of nitrogen in the tuberous root.
Nodulation and Nitrogen Fixation.
Despite
addition of 0.6% bagasse and consequent raising of the soil carbon-nitrogen
ratio, the products of nitrogen fixation, as estimated by
|
|
acetylene reduction, contributed only a
fraction of the total plant nitrogen. The soil at the experimental site was a
Typic Haplustoll and may be considered quite fertile. Various molar ratios of acetylene-reduced to nitrogen-fixed have
been described; generally these fall between
2.7 and 4.2 for soybean (Bergersen, 1970).
Assuming the ideal ratio of three acetylene molecules to each molecule
of atmospheric nitrogen reduced, and compensating for diurnal fluctuations in
nitrogenase activity (Chapter IV), the proportion of symbiotically-fixed
nitrogen to total plant nitrogen was calculated to be 9.2%, 4.5%, and 1.8% at
weeks 3.5, 6.5 and 12, respectively (Figure 13). However, total nodule recovery
was probably incomplete during the latest sampling.
Because this was a site
specific observation, additional studies must be conducted before the genetic
potential of P. erosus to provide its nitrogen requirement
through the root nodule symbiosis can be determined.
The nodule mass per plant
(Figure 14a) and the nodule specific activity (μ moles ethylene ·g nods-1
·hour-1)(Figure 14b) indicate that between weeks three and eight,
nodule mass increased linearly (r=0.98) from 0.10 to 0.52 grams of nodules per
plant. Between weeks eight and twelve,
both nodule mass and nitrogen fixation decreased. Standing water resulting from heavy rains and consequent
waterlogging probably was responsible for most of this decrease. This is in accord with observed reduction in
nodulation of Vigna unguiculata due to waterlogging (Minchin and
Summerfield, 1976). Also, increases in
tuberous root diameters as “bulking” proceeded resulted in the necrosis of many
of the root nodules as these nodules were spacially displaced and connective
tissues severed by the expanding tuberous root (Figure 15).
Nitrogenase specific activity did not vary
greatly between weeks three and seven, despite changes in nodule and root
morphology that took place at that time (Figure 16). However, the same factors which reduced nodule mass, particularly
oxygen stress, probably acted to reduce nodule specific activity
|
|
|
|
|
|
|
|
during the week 12
observation.
Effects of Pod Removal.
In P. erosus
root tuberization and seed development were competitive and substitutable
sinks. Total dry weight and tuberous
root weight were increased by flower bud removal (Table 13, Figures 17 and
18). The increase in dry weight of
tuberous roots due to flower removal was greater than the weight of the
reproductive parts of normal plants, indicating that some fraction of
assimilate that was diverted from pod fill promoted increased vegetative vigor,
which in turn resulted in increased dry weight of tuberous roots (Table 13).
Substituting a more
efficient sink for a less efficient one increased net assimilation in sugar beet
(Beta vulgaris (L.)) grafting experiments (Thorn and Evans,
1964), as well as in graft combinations between two subspecies of Beta vulgaris,
chard and sugar beet (Loomis et al, 1976).
Root tuberization may be a more efficient sink than seed development in P.
erosus, accounting for this increased dry matter when pods were
removed. Increased root tuberization in
response to periodic flower removal has been documented by Bala and Stephenson
(1978) and Herath and Fernandez (1978) in Psophocarpus tetragonolobus,
another tuberous-rooted legume.
Flower removal of P.
erosus resulted in significant increases in total nitrogen accumulation
in the roots (+ 3.0 kg N/ha) and shoots (+ 22.5 kg N/ha). Root nodule activity could not be assayed at
this late stage of root development, and since the root system of the
deflowered treatments was larger than that of the control, the effects of
increased nitrogen fixation and uptake of soil nitrogen could not be
separated. In soybean, pod removal has
also been shown to result in increased root weight (Loong and Lenz, 1974) as
well as increased nitrogen fixation (Lawn and Brun, 1974).
The nitrogen percentage
of the tuberous roots of P. erosus was not
|
|
|
|
significantly altered by
deflowering; therefore, unlike the nitrogen contained in the shoots, the
nitrogen stored in the tuberous roots was
not available to the
demands of later podfill. The nitrogen
percentage of the tuberous root declined somewhat at initiation of the inflorescences,
but remained constant thereafter (Figure 12).
Deflowering, and
consequent lack of assimilate diversion into the reproductive sink, did not
influence the concentration of total dissolved solids in the tuberous root
(Table 13). However, both total dry
matter and total dissolved solids per tuberous root were increased as a
function of increased tuberous root weight.
This could be an attractive and easy management practice since most of
the inflorescenses of P. erosus rise well above the leaf canopy
of unstaked plants.
The slight decrease in
the moisture content of the tuberous roots that resulted from deflowering was
significant. However, this 0.7%
difference does not nearly offset the increase in tuberous root yield.
Lateral symmetry of the
tuberous roots was decreased by deflowering. This could affect the efficiency
of mechanical harvesting schemes, but since these schemes do not presently
exist, this is of small consequence.
Much more serious would be loss of market appeal due to irregular
tuberous root shape.
Neither the frequency of
multiple tuberous roots per plant nor the cracking of tuberous roots were
related to flower removal. Multiple
tuberous roots per plant probably resulted from branching of the young tap root
apex. Since this occurred prior to flowering,
the removal of those flowers would not result in extra multiple tuberous roots.
Cracking of the tuberous
roots (Figure 19) was associated with heavy rainfall and standing water. During the eight days prior to harvest, 122
mm. of rainfall was recorded.
Pronounced lenticil development (Figure 20) preceded the cracking,
indicative of poor oxygen relations in the plant root. Cracking served to raise the ratio of root surface area to mass.
Exposed interiors of the tuberous roots callous quickly, but there was some
growth of saphophatic fungi. Planting
on raised beds should reduce the problem of tuberous root cracking under wet
soil conditions.
Cracking did reduce the
proportion of marketable tuberous roots in the deflowered treatment but total
marketable yield per unit area was still increased (Table 14). Total tuberous root and total marketable
tuberous root yield was increased by 16.2 metric tons/ha and 6.1 metric
tons/ha, respectively.
In conclusion, the
benefits of deflowering P. erosus as a photosynthetic source-sink
manipulation in the field increased yield and nitrogen accumulation. Problems associated with such treatment were
decreased root symmetry and moisture content.
These are relatively minor compared to the 33% yield increase of marketable
tuberous roots.
SUMMARY
Pachyrhizus erosus demonstrated phasic partitioning of dry matter
and nitrogen into shoots, followed by tuberous roots, followed by reproductive
structures. The nitrogen content of the
tuberous root remained constant during the period of root enlargement. P. erosus was shown to be a
nodulated legume, but its genetic potential to meet its nitrogen requirement
through symbiosis could not be determined in the present study. Deflowering P.
erosus resulted in increased root tuberization and nitrogen accumulation
and should be considered as a field scale management practice for the
production of tuberous roots of this legume.
|
|
|
|
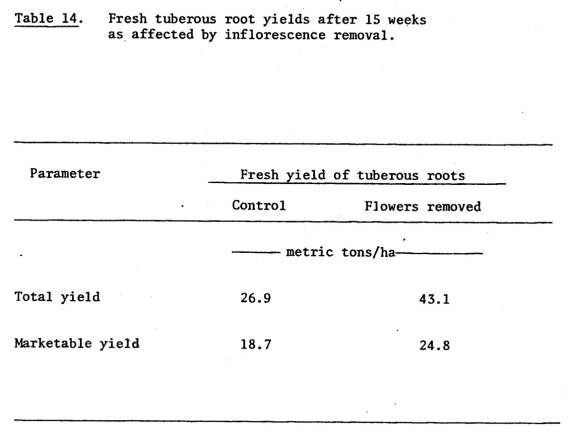
CHAPTER
VI
THESIS
SUMMARY
Several aspects of
symbiotic nitrogen fixation and root tuberization of Pachyrhizus erosus
(L.) (Mexican yam bean) were examined in the growth room glasshouse and field
at the NifTAL Project site, Paia, Maui.
A wide spectrum of Rhizobium
isolates were capable of nodulating P. erosus but only two of the
twenty three strains examined were able to establish highly effective symbiotic
relationships when compared to the nitrogen supplied control. The best symbiotic treatments assimilated
more nitrogen but less total dry matter than did the nitrate treatment.
The nitrogen nutrition of
the plant did not affect the proportional partitioning of dry matter into the
tuberous root during short days but the tissue concentration of nitrogen in the
tuberous root was influenced by both the form of available nitrogen and the
effectiveness of the root nodule symbiosis.
The extent of diurnal
fluctuation in symbiotic nitrogenase activity as measured by acetylene
reduction was not altered by changes in root morphology during root
tuberization. This aspect of
nitrogenase activity was no different than that of the normal rooted legume Vigna
unguiculata (L.) Walp. The ratio
of maximum to minimum activity varied from 1.4:1 to 1.9:1 under field conditions. Diurnal changes in nitrogenase were shown to
result from fluctuations in the environment, and not from an endogenous
rhythm. Prolonged darkness of 174 hours
reduced nitrogenase specific activity of tuberous-rooted plants to 40% of the
original dark period level. This was not direct evidence that the tuberous
root supports nitrogen fixation because other investigators have observed
similar results with normal rooted legumes.
Storage organ
accumulation was shown to follow the phasic pattern of partitioning. In the field, root tuberization and seed
development were equal, competitive sinks for carbon but the reproductive
structures competed more strongly for nitrogen than did the tuberous
roots. Thus inflorescences were not
able to exploit the positional advantage for fixed carbon and the tuberous root
was not able to exploit its advantage for assimilation of translocated
nitrogen.
Deflowering P. erosus
increased root tuberization and total root nitrogen and could be practiced on a
field scale since the inflorescences rise well above the unstaked leaf
canopy. The marketable yield of
tuberous roots after 15 weeks of growth was increased 30% by periodic flower
removal (24.8 and 18.7 metric tons/ha for deflowered and control treatments
respectively).
CHAPTER VII
LITERATURE CITED
1. Allen, 0. N., and Allen, E. K. 1939.
Root nodule bacteria of some tropical leguminous plants. II. Cross inoculation tests within the
cowpea group. Technical Paper number
115 of the Pineapple Research Institute (Reprinted from Soil Science 47(1)
January, 1939).
2. Anonymous.
1977. Statistics of Hawaiian
Agriculture. Hawaii Agricultural
Reporting Service.
3. Anonymous.
1979. A Planting Guide for
Vegetables in Hawaii. Hawaii
Cooperative Extension Service.
4. Ayanaba, A. and Lawson, T. L. 1977.
Diurnal changes in acetylene reduction of field grown cowpeas and
soybeans. Soil Biology and Biochemistry
9:125-129.
5. Bach, M. K., Magee, W. E., and Burris, R.
H. 1958. Tranlocation of photosynthetic products to soybean nodules and
their role in nitrogen fixation. Plant
Physiol. 40:118-124.
6. Bala, A. and Stephenson, R. 1978.
The genetics and physiology of tuber production in winged beans. Presented at the International
Workshop/Seminar on the Development of the Winged Bean, Los Banos,
Philippines. January (in press).
7. Balandreau, J. P., Miller, C.R. and
Dommergues, Y. R. 1974. Diurnal variations of nitrogenase activity
in the field. Applied Microbiology
4:662-665.
8. Bautista, 0. D. K. and Cadiz, T. G. 1967.
in Vegetable Production in S. E. Asia. J. Knott and J. Deanon (Editors) pp. 301-305.
9. Bergersen, F. J. 1970. The quantitative
relationship between nitrogen fixation and the acetylene reduction assay. Australian J. of Biological Sci.
23:1015-1025.
10. Bethlenfalvay, G. J., Abu-Shakra, S. S.,
Fishbeck, K. and Phillips, D.A. 1978.
The effect of source-sink manipulations on nitrogen fixation in peas. Physiologia Plantarum 43(1):31-34.
11. Bezdicek, D. F., Evans, D. W., Abede, B. and
Witters, R. E. 1978. Evaluation of peat
and granular inoculum for soybean yield and N fixation under irrigation. Agronomy Journal 70:865-868.
12. Bond, G. and Mackintosh, A. 1975.
Diurnal changes in nitrogen fixation in the root nodules of Casuarina. Proc. Royal Society London Bulletin
192:429-433.
13. Broughton, W. J. and Dilworth, M. J. 1971.
Control of leghemoglobin synthesis in snakebeans. Biochemistry Journal 125:1075-1080.
14. Brucher, H.
1976. Wild or semi-cultivated
leguminosae, rich in protein, from Latin-America and their nutritional
significance in the future (German).
Qualitas Plantarum.
26(1/3):71-106.
15. Burrill, T. J. and Hansen, R. 1917.
Is symbiosis possible between legume bacteria and non-legume plants? Illinois A.E.S. Bulletin No. 202:13-179.
16. Burton, J. C. 1977. Associations of
some uncommon leguminous plants with various rhizobia. in Exploiting the Legume-Rhizobium
Symbiosis in Tropical Agriculture.
J. M. Vincent, A. S. Whitney and J. Bose (Editors) pp. 381-385.
17. Ching, T. M., Hedtke, S., Russell, S. A. and
Evans, H. J. 1975. Energy state and dinitrogen fixation in
soybean nodules of dark-grown plants. Plant Physiol. 55:796-798.
18. Ciha, A. J., and Brun, W. A. 1978.
Effect of pod removal on nonstructural carbohydrate concentration in
soybean tissue. Crop Science
18:773-776.
19. Coursey, D. G., and Haynes, P.H. 1970.
Root crops and their potential as food in the tropics. World Crops. 22:261-265.
20. Das Gupta, D. K. 1969. The effect of
decapitation on growth of the sugar beet storage root. in Root Growth. W. J. Whittington (Editor) pp. 247-255.
21. Degras, L. M. 1967. Growth and storage
in tropical root crops. Proc.
International Symposium on Tropical Root Crops. E. A. Tai, et al. (Editors) Volume 3, pp. 18-25.
22. Deshaprabhu, S. B. (Editor). 1966. Pachyrhizus spp. The Wealth of India: Raw Materials. Vol. 7, (N-Pe) pp. 208-210. New Delhi, Public Information Council.
23. Duhigg, P., Melton, B. and Baltensperger, A.
1978.
Selection for acetylene reduction rates in “Mesilla” alfalfa. Crop Science 18:813-816.
24. Evans, M., Boulter, D., Eaglesham, A.R.J.
and Dart, P.J. 1977. Protein content and protein quality of
tuberous roots of some legumes determined by chemical methods. Qualitas Plantarum 27(3-4):275-285.
25. Ezumah, H.
1970. Miscellaneous tuberous
crops of Hawaii. Proc. Second
International Symposium of Tropical Root Crops. D. Plucknett (Editor) pp. 166-171
26. Fishbeck, K., Evans, H. and Boersma, L. 1973.
Measurement of nitrogenase activity of intact legume symbiosis in
situ, using the acetylene reduction assay. Agronomy Journal 65:429-433.
27. Garner, W. W. and Allard, H. A. 1923.
Further studies in photoperiodism. Journal of Agricultural Research. 23:871-920.
28. Greig, J. J., Pate, J. S. and Wallace,
W. 1962. Techniques for studying physiological rhythms in legume
symbiosis. Life Sciences 12:745-752.
29. Hardy, R.W.F., Holsten, R. D., Jackson, E.
K. and Burns, R. C. 1968. The acetylene-ethylene assay for N2
fixation: laboratory and field evaluation.
Plant Physiol. 43:1185-1207.
30. Hardy, R.W.F., Burns, R. C. and Holsten, R.
D. 1973. Application of the acetylene-ethylene assay for measurement of
nitrogen fixation. Soil Biology and
Biochemistry 5:47-81.
31. Haynes, P. H., Spence, J. A., and Walter, C.
J. 1967. The use of physiological studies in the agronomy of root crops. Proc. International Symposium on Tropical
Root Crops. E. A. Tai, et al. (Editors)
Volume 3, pp. 1-18.
32. Herath, H.M.W. and Fernandez, G.O.J. 1978.
Effect of cultural practices on the yield of seed and tubers in winged
beans (Psophocarpus tetragonolobus).
International Workshop/Seminar on Development of the Winged Bean. Los Banos, Philippines. January (in press).
33. Jeffers, J.P.W. 1976. Mechanization of
yam and sweet potato production in Barbados.
Proc. Fourth International Symposium on Tropical Root Crops. J. Cock, et al. (Editors) pp. 275-277.
34. Johnston, B. 1967. The discrepancy between
social and private returns to mechanization in early phase of economic
development. Proc. International
Symposium of Tropical Root Crops. E.A.
Tai, et al. (Editors) Volume 5:67-71.
35. Kay, D. E.
1973. Root Crops. Tropical Products Institute, 56/62 Grays Inn
Road, London. pp. 240-245.
36. Lawhead, C. W. 1978. Effects of
photoperiodism and growth regulators on reproductive and vegetative aspects of
the winged bean, Psophocarpus tetragonolobus (L.) DC. Thesis submitted March 1978, Dept. of
Vegetable Crops, University of California at Davis.
37. Lawn, R. J. and Brun, W. A. 1974.
Symbiotic nitrogen fixation in soybeans. I. Effect of photosynthetic source-sink manipulations. Crop Science 14:11-16.
38. Lawrie, A. C. and Wheeler, C. T. 1973.
The supply of photosynthetic assimilates to nodules of Pisum sativum
(L.) in relation to the fixation of nitrogen.
New Phytologist 72:1341-1349.
39. Lawrie, A. C. and Wheeler, C. T. 1975.
Nitrogen fixation in the root nodules of Vicia faba (L.)
in relation to the assimilation of carbon.
I. New Phytologist 74:427-436.
40. Lawrie, A. C. and Wheeler, C. T. 1976.
Nitrogen fixation in root nodules of alder and pea in relation to the
supply of photosynthetic assimilates. Chapter 34 in Symbiotic Nitrogen
Fixation in Plants, P. S. Nutman (Editor).
41. Leslie, K.
1967. The significance of root
crops in the tropics. Proc.
International Symposium on Tropical Root Crops. E. A. Tai, et al. (Editors) Volume 5, pp. 1-13.
42. Lindstrom, E. S., Newton, J. W. and Wilson,
P. W. 1952. The relationship between photosynthesis and nitrogen
fixation. Proceedings of the National
Academy of Science 38:392-396.
43. Loomis, R. S., Ng, E., and Hunt, W. F. 1976.
Dynamics of development of crop production systems. CO2 Metabolism and
Plant Productivity. Burris, R.
H. and Black, C.C. (Editors), Univ. Park Press, Baltimore, Md. pp. 269-286
44. Loomis, R. S. and Rapoport, H. 1976.
Productivity of Root Crops.
Proc. Fourth International Symposium on Tropical Root Crops, pp. 70-84.
45. Loong, S. G. and Lenz, F. 1974.
Effects of nitrogen level and fruit removal on growth, nodulation and
water consumption of soybean. Z.Acker-und Pflanzenbau 139:35-43.
46. Mague, T. H. and Burris, R. H. 1972.
Reduction of acetylene and nitrogen by field grown soybeans. New Phytologist 71:275-281.
47. Maitra, S. and Deepesh, N. 1971.
Role of microtubules in secondary thickening of differentiating xylem
elements. J. Ultrastructural Research
34:15-22.
48. Marcarian, B. 1978. in Feasibility
of Introducing Food Crops Better Adapted to Stress. National Science Foundation, pp. 131-133.
49. Mederski, H. J. and Streeter, J. G. 1977.
Continuous, automated acetylene reduction assays using intact soybean
plants. Plant Physiol. 59(6):1076-1081.
50. Mes, M. G.
1959. Influence of temperature
on the symbiotic nitrogen fixation of legumes.
Nature (London) 184:2032-2033.
51. Milthorpe, F. L. 1967. Some physiological
principles determining the yield of root crops. Proc. International Symposium on Tropical Root Crops. E. A. Tai, et al. (Editors) Volume 2, pp.
1-19.
52. Minchin, F. R., and Pate, J. S. 1974.
Diurnal functioning of the legume root nodule. J. of Experimental Botany 25(85): 309-319.
53. Minchin, F. R. and Summerfield, R. J. 1976.
Symbiotic nitrogen fixation and vegetative growth of cowpea (Vigna
unguiculata (L.) in waterlogged conditions. Plant and Soil 45:113-127.
54. Mitchell, H. L. 1972. Microdetermination
of nitrogen in plant tissues. J. of the Assoc. of Official Analytical Chemists
55:1-3.
55. Mondal, M. H., Brun, W. A. and Brenner, M.
L. 1978. Effects of sink removal on photosynthesis and senescence in
leaves of soybean (Glycine max L.) plants. Plant Physiol.
61:394-397.
56. National Academy of
Science. Tropical Legumes_- a
Neglected Resource (in press).
57. Pate, J. S.
1962. Nodulation studies in
legumes. V. The effects of temperature
on symbiotic performances of bacterial associations of Medicago tribuloides
(Desr.) and Vicia atropurpurea (Desf.) Phyton (Buenos Aires)
18(1):65-74.
58. Pate, J.S.
1976. Physiology of the reaction
of nodulated legumes to environment.
Chapter 27 in Symbiotic Nitrogen Fixation in Plants. P. S. Nutman
(Editor).
59. Pate, J. S. and Greig, J. M. 1964.
Rhythmic fluctuations in the synthetic activities of the nodulated root
of the legume. Plant and Soil
21(2):163-184.
60. Pate, J. S., Gunning, B.E.S. and Briarty, L.
G. 1969. Ultrastructure and functioning of the transport system of the
legume root nodule. Planta (Berlin) 85:11-34.
61. Purseglove, J. W. 1968. in Tropical
Crops, Dicotyledons. Longman Group,
Ltd., London. pp. 281-284.
62. Rachie, K. 0. and Roberts, L. M. 1974.
in Grain Legumes of the Lowland Tropics. Advances in Agronomy, Volume 26 of the
Academic Press, Inc. pp. 90-91.
63. Ruegg, J. J. and Alston, A. M. 1978.
Seasonal and diurnal variation of nitrogenase activity in barrel medic
grown in pots. Aust. J. Agric.
Res. 39:951-962.
64. Shaposnikov. 1975. Daily changes in
the rate of molecular nitrogen fixation, free amino acid content and ammonia
content in Lupin nodules. Soviet Plant Physiology 22:681-688.
65. Sinclair, A. G., Hennagan, R. B. and
Johnstone, P. 1978. Evaluation of a non-destructive acetylene
reduction assay of nitrogen fixation for pasture legumes grown in pots. N.Z.J. of Experimental Agriculture 6:65-68.
66. Sloger, C., Bezdicek, D., Milberg, R., and
Boonkerd, N. 1975. Seasonal and diurnal variations in N2-fixing
activity in field soybeans. Nitrogen Fixation by Free Living Microorganisms, W.
Stewart (Editor).
67. Spratt, E. R. 1919. A comparative
account of the root nodules of the Leguminosae. Ann. of Botany 33(80):189-199.
68. Sprent, J. I. 1969. Prolonged reduction
of acetylene by detached soybean nodules.
Planta (Berlin) 88:372-375.
69. Thorn, J. H. and Evans, A. F. 1964.
Influence of tops and roots of net assimilation rate of sugar beet and
spinach beet and grafts between them.
Ann. Botany 28:499-508.
70. Torrey, J.
1976. Root hormones and plant
growth. Ann. Rev. Plant Physiol. 27:435-459.
71. Trinick, M. J., Dilworth, M. J. and Grounds,
M. 1976. Factors affecting the reduction of acetylene by root nodules of Lupinus
species. New Phytologist
77:359-370.
72. Vincent, J.
1970. A manual for the practical
study of the root nodule bacteria. IBP
Handbook 15.
73. Virtanen, A. I., Moisio, T. and Burris, R.
N. 1955. Fixation of nitrogen by nodules excised from illimunated and
darkened pea plants. Acta. Chem. Scand.
9:184-186.
74. Walker, R. H. 1928. Physiological
studies on the nitrogen fixing bacteria of the genus Rhizobium. Iowa A.E.S.
Research Bulletin No. 113:371-406.
75. Weaver, R.
1975. Growing plants for Rhizobium
effectiveness tests. Soil Biology and Biochemistry. 7:77-78.
76. Wheeler, C. T. 1969. The diurnal
fluctuation in nitrogen fixation in the nodules of Alnus glutosa
and Myrica gale. New Phytologist 68:675-683.
77. Whiteman, P. C. 1970. Seasonal changes in
the growth and nodulation of perennial pasture legumes in the field. III. Effects of flowering on nodulation of
three Desmodium species. Aust.
J. Agr. Res. 21:215-219.
78. Wilson, P. W., Fred, E. B. and Salmon, M.
R. 1933. Relation between carbon dioxide and elemental nitrogen
assimilation in leguminous plants. Soil
Science 35:145-165.
|
|
APPENDIX I
|
|
|
|
APPENDIX 3
APPENDIX 4
|
|
APPENDIX 5
|
|